g/L is a unit of measurement of mass concentration that shows how many grams of a certain substance are present in one liter of liquid. Molarity (M) is the concentration of a solution expressed as the number of moles of solute per litre of solution. So, two substances with a same g/L can be different in Molarity.
Just so, How do I convert GL to mol L?
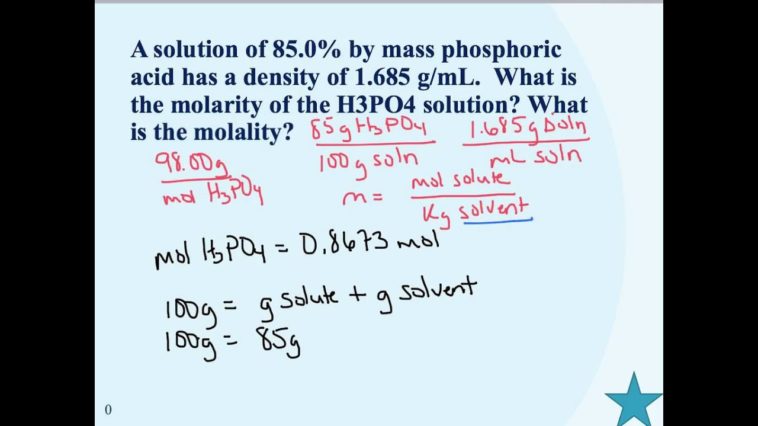
How do you calculate molarity from GL?
Similarly, What is mg N L?
( mass of nitrate ions per liter) Or in terms of a particular element in a species that was measured. e.g., mg/L of NO3. – – N (mass of nitrogen in the form nitrate ions per liter)
How do you calculate molarity from GL?
Is mg/ml the same as G L?
mg/mL↔g/L 1 mg/mL = 1 g/L.
How do I find my GL 1?
Calculate the concentration using c = n/V (remember V is always in litres) L. To convert to g L-1 you multiply the concentration by the molar mass; = 0.118 mol L-1 x 55.9 g mol-1 = 6.60 g L-1 (3 s.f.) Note: If you calculate this keeping all the figures in your calculator from step 4 you will get 6.58 g L-1 (3 s.f.)
What does 1 molar solution mean?
Molarity is another standard expression of solution concentration. Molar solutions use the gram molecular weight of a solute in calculating molar concentration in a liter (L) of solution. … A 1 molar (M) solution will contain 1.0 GMW of a substance dissolved in water to make 1 liter of final solution.
How do you convert molar concentration to GL?
- FW of NaCl : 23+35.45 = 58.5.
- 0.9 % (w/v) is 0.9 g/100 ml = Mass conc. 9 g/l.
- Molar concentration.
- = Mass conc. ( g/l) / FW.
- = 9/58.5 = 0.15 mol/l.
What does GL 1 mean?
GL1
| Acronym | Definition |
|---|---|
| GL1 | Gliese 1 (red dwarf star) |
How do you convert mg/mL to GL?
mg/mL↔g/L 1 mg/mL = 1 g/L. mg/mL↔kg/m3 1 mg/mL = 1 kg/m3.
Is mg/kg same as mg L?
they are the same. If you know the density of the solution expressed in kg/l, you can divide your value in mg/l by the density so that (mg/l) / (kg/l) results in a value in mg/kg.
How do you find molarity with L and G?
- First you must calculate the number of moles in this solution, by rearranging the equation. No. Moles (mol) = Molarity (M) x Volume (L) = 0.5 x 2. = 1 mol.
- For NaCl, the molar mass is 58.44 g/mol. Now we can use the rearranged equation. Mass (g) = No. Moles (mol) x Molar Mass (g/mol) = 1 x 58.44. = 58.44 g.
How do you calculate concentration from GL?
Divide the mass of the solute by the total volume of the solution.
- In our example for the concentration of 3.45 grams of salt in 2 liters of water, your equation would be C = (3.45 g)/(2.002 L) = 1.723 g/L.
- Certain problems may ask for your concentration in specific units.
Is mg L same as PPM?
PPM and mg/L are two different measures of substance concentration. … PPM is the number of salt parts per a million parts of the entire solution, both water and salt. mg/L, or milligrams per liter, is a measure of concentration.
What measurement is g l?
Unsourced material may be challenged and removed. A gramme per litre or gram per liter (g/L or g/l) is a unit of measurement of mass concentration that shows how many grammes of a certain substance are present in one litre of a (usually liquid or gaseous) mixture.
How do you convert GL to ML?
Please provide values below to convert gigaliter [GL] to milliliter [mL], or vice versa.
…
Gigaliter to Milliliter Conversion Table.
| Gigaliter [GL] | Milliliter [mL] |
|---|---|
| 0.1 GL | 100000000000 mL |
| 1 GL | 1000000000000 mL |
| 2 GL | 2000000000000 mL |
| 3 GL | 3000000000000 mL |
How much is 5 liters in milligrams?
How Many Milligrams are in a Liter?
| Volume in Liters: | Weight in Milligrams of: | |
|---|---|---|
| Water | Cooking Oil | |
| 0.004 l | 4,000 mg | 3,520 mg |
| 0.005 l | 5,000 mg | 4,400 mg |
| 0.006 l | 6,000 mg | 5,280 mg |
What is 0.1N solution?
The normality of a solution is the gram equivalent weight of a solute per liter of solution. … For example, the concentration of a hydrochloric acid solution might be expressed as 0.1 N HCl. A gram equivalent weight or equivalent is a measure of the reactive capacity of a given chemical species (ion, molecule, etc.).
What does a 1 molar solution contain?
A molar solution is defined as an aqueous solution that contains 1 mole (gram-molecular weight) of a compound dissolved in 1 liter of a solution. In other words, the solution has a concentration of 1 mol/L or a molarity of 1 (1M).
How do you make a 0.5 molar solution?
For example, if you wanted a 0.5 M solution, you would use 0.5 x 58.44 g/mol of NaCl in 1 L of solution or 29.22 g of NaCl.